Alkaloids are a chemically heterogenous group of natural substance and compose more than 6000 basic Nitrogen containing organic compounds which occur in about 15% of all vascular terresterial plants and in more than 150 different plant families. The alkaloids exhibit diversity of structure and also show an extraordinary spectrum of pharmacological activities. Because of these characters, they are important for chemical, physiological studies, taxonomical studies and biogenetic studies.Introduction Two main source of medicine are, one is synthetic and another
is naturally occurring. Synthetic drugs show rapid onset of action but having
more side effects in comparison to naturally occurring drugs. The modern
trend are back to choose natural medicine against synthetic medicine. Natural
source of drugs are plants, animals, or minerals. About 8000 plants are listed
in medicinal uses. In this 1800 in Ayurveda, 1100 in Shiddha, 750 in Unani,
300 in Tibetan and 4700 plants are used as traditionally ethno medicinally.
The world over the total trade of medicinal plants about 8800-lakh
dollar, of which contribution of India in world trade less then 1%.
Out of this majority of plants are yet to be study photochemical, estimated of
such pants to the extent of 5000 to 6000. Phytochemistry is branch of Chemistry, which deals with the study of
chemistry of plants. Where the term phytochemistry is comes out from phyta
+ chemistry (phyta plant).Pharmaphytochemistry The word pharmaphytochemistry is derived from
pharmakon drugs phyta plants i.e. chemistry of medicinal substance
inside the plants.Alkaloids Chemistry: Sertuerner in 1806 laid the foundation of Alkaloids
Chemistry. It is the branch of Pharma Phyto Chemistry, which deals with the
study of Alkaloids. He reported isolation of Morphine from opium.
What is Alkaloids: Alkaloids means Alkali likes. The Pharmacist
W.Meissner proposed the term Alkaloids in 1819. Acc. to him "Alkaloids
(alkali = base, oid=like sub) are basic nitrogenous compd. of plant origin
which have complex molecular structure & many pharmacological activity."
Acc to Landenberg "Alkaloids are defined as natural plant compounds
that have a basic character and contain at least one nitrogen atom in a
heterocyclic ring and having biological activities."Acc to characteristic features Alkaloids are basic nitrogenous plant
origin, mostly optically active & possessing nitrogen hetero cycles as there
structural units with physiological action.

The term alkaloids or Pflanzenlkalien was coined by Meissner, a German pharmacist, in 1819. the mankind has been using alkaloids for various purposes like poison, medicine, poultices, teas, etc
The French chemist, Derosne in 1803, isolated narcotine. In the same year, morphine from opium was isolated by Serturner, a pharmacist of Padeborn near hannover in 1803. Pelletier and Caventon from the Faculty of pharmacy of Paris isolated emetine in 1817 and colchicines in 1819. this was followed by isolation of series of alkaloids from vegetable drug, like strychnine (1817); brucine, piperine and caffine (1819), quinine, colchicines and cinchonine (1820); coniine (1826), papaverine(1821) and thebaine (1835). By 1884, about 25 alkaloids were reported to be isolated from cinchona bark alone. But in 1870, a landmark in domain of alkaloid was achieved by determining thestructure of coniine, which also become first synthesized alkaloid in 1889. From the beginning of 19th century till to date, it has proved to be a perpetual work of discover new alkaloids from the plant and animal. In the present century, the proper structure were assigned to various alkaloids with the help of chromatographic and another sophisticated physical method of analysis. As per a Russian review in 1973, the number of known alkaloids had reached upto 4959, amongst which, the structure of 3293 alkaloids were elucidate. At present, the number of alkaloids discovered has exceeded 6000.
In the view of their chemical and physiological diversity, there is no comprehensive definition of alkaloids. The term is derived from the word alkali-like and hence, they resemble some of the characters of naturally occurring complex amines. The term alkaloids also cover proto alkaloids and pseudoalksloids. In view of all such variations, the only definition that bring all such compounds under one title is as follows:
These are the organic product of natural and synthetic origin which are basic in nature and contain one or more than one nitrogen compound normally in heterocyclic in nature, and posses specific physiological action on human and animal body, when use in small quantities
The true alkaloids are toxic in nature and contain one or more heterocyclic nitrogen compound which is derived from amines and always basic in nature. True alkaloids are normally present in plants as salts of organic acids. The proto-alkaloids or amino-alkaloids in which nitrogen is not present in heterocyclic ring.
Some times they are considered as biological amines. They are basic in nature and prepared in plants from amino acids. Some of the examples of these alkaloids are mescaline, N-dimethyl tryptamine, colchine and ephedrine. The term pseudoalkaloid includes mainly steroidal terpenoides alkaloids and purine. They are not derived from amino acids. They do not show many of typical characters of alkaloids, but give the standard qualitative test for alkaloids. The example of pseudoalkaloids are conessine and caffine.
Alkaloids are chemically nitrogenous heterocyclic basic compound occur in
nature, about15% of vascular plant & widely distributed in higher plant e.g.. -
Apocynace, papaveraceae, papilanaceae, rananeulaceae, solenaceae.
They are present in the form of salts of organic acid, like acetic acid,
oxalic acid, malic, lactic, tartaric, tannic, aconitic acid, few are with sugar e.g.
Solanum, veratrum groups. Acc. to parts of plants:
Leaves: Nicotine
Bark: Cinchonine, Quinine.
Seeds: Strychnine, Nibidine.
Roots: Rawelfinine, Glycyrrhizin, Punarnavine I & II
There was no systematic nomenclature. But there are some methods for
nomenclature are mention below.
- According to their source:
- There are named according to the family in which they are found e.g. papavarine, punarnavin, ephedrin.
- According to their Physiological response:
- There are named according to their physiological response e.g.. Morphine means God of dreams, emetine means to vomit.
- According to there discover:
- There are named according to there discover e.g.. pelletierine group has been named its discoverer, P.J. Pelletier.
- Prefixes:
- There are named by some prefixes are fix in nomenclature of alkaloids, e.g. epi, iso, neo, pseudo, nor- CH3 group not attach to Nitrogen 4
- PHYSICAL PROPERTIES
With few exemptions, all the alkaloids are colourless, crystalline solids with a sharp melting points or decomposition range. Some alkaloids are amorphous gum, while other coniine, spartine, nicotine etc. are liquid and volatile in naruer. Some alkaloids are coloured in nature, eg. Betanidin is red, berberine is yellow and salts are copper-red in colour.
In general, the free bases of alkaloids are soluble in organic non-polar, immiscible solvents. The salts of more alkaloids are soluble in water. In contrast, free bases are insoluble in water and their salts are also sparingly soluble in organic solvents. The alkaloids containing quaternary bases are only water soluble. Some of the pseudoalkalods and protoalkaloids shows higher solubility in water. For examples, colchicines is soluble in alkaline water, acid and water and caffeine (free base) is freely soluble in water. Quinine hydrochloride is highly soluble in water i.e. 1 part of quinine hydrochloride is soluble in less than 1 part of water, while only 1 part of quinine sulphate in 1000 parts of water.
The solubility of alkaloids and salts is useful in pharmaceutical industry for the extraction and formulation of final pharmaceutical properties
- CHEMICAL PROPERTIES
- Test by Dragendorff reagent(Potassium-bismuth-iodide solution) : -
- Alkaloids give reddish-brown precipitate with this reagent.
- test by Mayer reagent (Potassium-mercuric-iodide solution): -
- Alkaloids gives cream colour precipitate with this reagent.
- test by Wagner reagent (iodine-potassium-iodide solution): -
- Alkaloids give Brown colour precipitate with this reagent.
- test by Hager reagent (Saturated solution of picric acid): -
- alkaloids give yellow colour precipitate with this reagent.
- Test by Tannic acid: -
- Alkaloids gives buff colour precipitate with this acid
- Test by Picrolonic acid: -
- Alkaloids give yellow colour precipitate with this acid
PHARMACOLOGICAL CLASSIFICATION: -
- Depending on the physiological response, the alkaloids are classified under various pharmacological categories, like central nervous system stimulants or depressants, symphatomimentics, analgesic, purgative, etc. this method dose not take into account chemical nature of crude drugs with in the same drugs, the individual alkaloid may exhibit different action e.g. morphine is narcotic analgesic, while codenine is mainly antiussive. In cinchona, quinine is antimalarial, while quinidine is cardiac depressant.
TAXONOMIC CLASSIFICATION: -
- This method classifies the vast number of alkaloids based on their distribution in various plants families, like solanaceous or papillionaceous alkaloids. Preferably, they are grouped as per the name of genus in which they occur, e.g. ephedra, cinchona, etc. from this classification the chemotaxonomic classification has been further derived.
CHEMICAL CLASSIFICATION: -
- This is a most accepted way of classification of alkaloids. The main criterion for chemical classification is the type of fundamental(normally heterocyclic) ring structure present in alkaloids. The alkaloidal drugs are broadly categorized into two divisions.
- True alkaloids(heterocyclic alkaloids) are divided into twelve group according to the nature of their heterocyclic ring
- Protoalkaloids or biological amine and pseudoalkaloids.
TAXONOMIC BASED ON THEIR FAMILY: -
- e.g. Solanaceous,Papilionaceous without reference their chemical type of alkaloids present & another according to genus. e.g.. ephedra, cinchona etc. Pharmacological based: Their pharmacological activity or response. For example:
1. Analgesic alkaloids
2. Cardio active alkaloids etc. Do not have chemical similarity in their group. BIO SYNTHETIC CLASSIFICATION: -
- Pyridine group: piperine,coniine,trigonelline,arecaidine,guvacine,pilocarpine,cytisine,nicotine,sparteine,pelletierine.
- Pyrrolidine group: hygrine,cuscohygrine,nicotine
- Tropane group: atropine, cocaine, ecgonine, scopolamine, catuabine
- Quinoline group: quinine, quinidine, dihydroquinine, dihydroquinidine, strychnine,brucine, veratrine, cevadine
- Isoquinoline group: The opium alkaloids (morphine, codeine, thebaine, Isopapa-dimethoxy-aniline, papaverine, narcotine, sanguinarine, narceine, hydrastine, berberine), emetine, berbamine,oxyacanthine
- Phenethylamine group: mescaline, ephedrine, dopamine, amphetamine
- Indole group:
- Tryptamines: DMT, N-methyltryptamine, psilocybin, serotonin
- Ergolines: the ergot alkaloids (ergine, ergotamine, lysergic acid, LSD etc.
- Beta-carbolines: harmine, harmaline, yohimbine, reserpine
- Rauwolfia alkaloids: Reserpine
- Purine group:
- Terpenoid group:
- Aconite alkaloids: aconitine
- Steroids: solanine, samandaris (quaternary ammonium compounds): muscarine, choline, neurine
- Vinca alkaloids: vinblastine, vincristine. They are antineoplastic and binds free tubulin dimers thereby disrupting balance between microtuble polymerization and delpolymerization resulting in arrest of cells in metaphase.
- Miscellaneous: capsaicin, cynarin, phytolaccine, phytolaccotoxin
Chemical classification:
- This classification is universally adopted & depends
on the fundamental ring structure. According to these two main groups.
1. Non-heterocyclic Alkaloids: In this group of alkaloid not has any one
Heterocyclic ring in their structure. e.g.- Hordinine (Hordeum vulgare),
Ephedrine(Ephedra gerardiana) Genateceae.
2. Heterocyclic Alkaloids: According to heterocyclic ring the alkaloids are
sub divide in following: - - PYRROLE
- : This type of alkaloids contains pyroll or pyrrolidine ring in their structure e.g.. Hygrines Coca sp. For detailed information click here

- PYRROLIZIDINE
- Alkaloids containing Pyrrolizidine Heterocyclic ring in their structure e.g.. - seneciphylline Senecio sp. For detailed information click here

- PYRIDINE & PIPERIDINE
- Alkaloids containing Pyridine Heterocyclic ring in their structure e.g. Nicotine, Lobaline, Piperidine, Ricinine. For detailed information click here&click here
 &
&
- PIPERIDINE(TROPANE)
- Alkaloid containing tropone ring. e.g..-Hyoscyomine, Atropine Hyoscine- Solanceae Cocain- Coca sp. Tropane Quinoline Iso Quinoline Nor Lupinane Iodole For detailed information click here or tropane

- QUINOLINE
- Those Alkaloids containing quinolin ring in their structure e.g..- Quinine, Quinidine. (Cinchona bark) Cinchonine, Cinchonidine & cusparin -(cusparia bark) For detailed information click here

- ISO QUINOLINE
- Alkaloids containing iso quinoline ring in thier chemical structure e.g. Papavarine, Narceine Emetine & cephaline. (Cephalis sp Rubiaceae). For detailed information click here
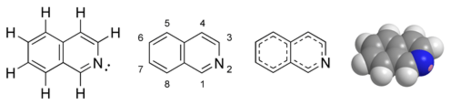
- REDUSED ISO QUINOLINE(APORPHINE)
- The alkaloid contain reduced isoquinoline ring in their structure e.g. Baldine, (Peumus Baldus) (Manioniaceae) For detailed information click here

- NOR LUPINANE
- Alkaloids present in leguminoceae plants e.g. spartine, lupanine.
- INDOLE ALKALOIDS
- Alkaloids containing indole ring. e.g. Yohimbine, Aspidospermine (Apocynaceae) Vinblasine, vincristine (catheranthus roseus). For detailed information click here

- Detection of presence of Alkaloids:
First of all confirm the presence of
alkaloids in raw material or crud drugs by various reagents called Alkaloids
reagents e.g.
a,Mayer (Cream Co lour) Test
b.Marquis (Conc. HCHO) Test.
c.Erdmann (Conc. HNO3) Test
d.Hager's (Yellow Colour) Test
e.Frohdes (Molybdic acid) Test
- Extraction: -
The plants is dried, then finally powdered and extracted with
boiling methanol. The solvent is distilled off and the residue treated with
inorganic acids, when the bases (alkaloids) are extracted as their soluble salts.
The aqueous layer containing the salt of alkaloids and soluble plant impurities
is made basic with NaOH. The insoluble alkaloids are set free precipitate out.
The solid man (ppt.) so obtained is then extracted with ether when alkaloid
pass into solution and impurity left behind. Flow Chart of extraction: -- Separation of Alkaloids:
- After detection of next step is separation of a relatively small percentage of alkaloids from large amount of crude drugs. E.g.- Opium contains 10% Morphine, Chincona contains 5-8 % Quinine, Belladona- 0.2% of Hyoscyamine.
The required alkaloid is separated from the mixture from fractional, crystallization, chromatography and ion exchange method. - PHYSICAL PROPERTIES
- They are colorless, crystalline solid. Exception - Berberin (Yellow), Nicotine Coniine (liquid).
- They are insoluble in water (exception liquid alkaloids soluble in water), soluble in organic solvent ( CHCl3, Ethyl alcohol ether)
- Taste: They are bitter in taste.
- Optically active, Most of levo ratatory but few are -Dextro rotatory e.g. Coniine, some inactive- e.g.- papaverine.
- Molecular formula molecular weight:
- A pure specimen of alkaloids its empirical formula and molecular weight by elemental or combustion analysis. No. Of double bond is determined by double bond equivalent method.
- Number of Double bond: -
- Number of Rings present in an alkaloids can be determine by following formula- Ca Hb Nc Od Then number of double bond present in Ring= a-b/2 + C/2 + 1
- Functional group Analysis:
- 1. Functional Nature of Oxygen: -
- Oxygen presents in alkaloids as: -
OH (Phenolic/ Alcoholic), - OCH3 Methoxy, - OCOCH3 (Acetoxy), - OCOC6H5 (Benzoxyl), -COOH (Carboxylic),- COOK (carboxylate),>C=O (Carbonyl),=C-O-O (Lactones Ring).It can be determined by infra red or organic analysis method. - 2. Hydroxyl group: -
- Formation of Acetate on treatment with Acetic anhydride /Acetyl chloride or benzoate on treatment with Benzyl chloride.
- Soluble in NaOH
- Re precipitated by CO2
- Giving coloration with FeCl3
- 3. Carboxylic group: -
- Soluble in aqueous solution sodium carbonate or ammonia on treat with alcohol form ester.Number of -COOH group can be determined by volumetrically by titration against a standard. Ba(OH)2 solution by using phenolphthalein as an indicator.
- 4. Oxo-group: -
- On treatment with Hydroxylamine. Semicanbezide, phenylhydrazide ,oxime ,semicarbazone phenyl Hydrazine
- 4. Methoxyle group: -
- BY Zeisel determination method. When methoxy group present in a alkaloids treated with HI at 126 0 C perform methyl iodide which can treated further with silver nitrites to perform silver iodide precipitate. Which estimated gravimetrically e.g.. Papavarine.
- 5. Methylenedioxy group: -
- On heated with concentrated with HCL or H2SO4 to form formaldehyde and further formation of dime done derivative, which estimated gravimetrically.
- b) Ester Amide lacton & Lactum group:
- These groups are identified by the estimation of product.
- c) Nature of Nitrogen
- Majority of nitrogen presence in alkaloids are secondary and tertiary: If tertiary when treated with H2 O2 (50%) form.
- 1.DIRECT CRYSTALLISATION FROM SOLVENT: -
- it is a very simple method of isolation and may not be useful in case of complex mixture
- 2. STEAM DISTILLATION: -
- This method is espateially employed for volatile liquid alcohols like coniine, sparteine, and nicotine, but other wise this process is not suitable for alcohols with high molecular weights.
- 3. CHROMATOGRAPHY TECHNIQUES: -
- This method has provided to be ideal for separation of a vast number of plant alkaloids. The different technique of chromatography (thin layer, column, gas, liquid, ion exchange chromatography, HPTLC, etc.) are used for separation of indivisual alkaloids from complex mixture.
- 4. GRADIENT pH TECHNIQUES: -
- Tthough alkaloids are basic in nature, there are variations in the extent in the basicity of various alkaloids of the same plant. Depending on this character, the crude alkaloidal mixture is dissolved in 2% tartaric acid solution and extracted with benzene so that the first fraction contains natural and/or very weakly basic alkaloids. pH of the aqueous solution is increased gradually by 0.5 increment upto pH 9, and extraction is carried out at each pH level with organic solvent, by this way alkaloids with different basicity are extracted. Strongly basic alkaloids are extracted at the end.
- They are the reserve substance with an ability to supply nitrogen.
- They might be the defensive mechanism for plant growing in dry regions to protect from grazing animals, herbivores and insects.
- It is also possible that they are end product of detoxification mechanism in plants, and by this way check formation substance which may prove to be harmful to the plants.
- They might have a possible role as growth regulatory factors in plants
- The are present normally in conjugation with plants acids, like meconic acid, cinchotannic acid etc. there foe alkaloids could be acting as carriers with in plants for transportation of such acids.
Most of the alkaloids are basic in reaction, due to availability of lone pair of electron on the nitrogen ring. The basic character of the alkaloidal compound is enhanced if the adjust function groups are electron releasing. The alkaloid turns to be natural or acidic when the adjust functional groups are electron withdrawing like amide group which reduces availability of lone pair of electrons. But alkaloids exhibits basic characters are very much sensitive to decomposition and cause a problem during their storage. Their salt formation with inorganic acid prevents many a time their decomposition.
The alkaloids may contain one or more number of nitrogen and it may exist in the form as primary(R-NH2), eg. Mescaline, secondary amine (R2-NH),eg.ephedrine; tertiary amine(R3-N),eg. Atropine; and quaternary amine (R4N+X),eg. Tubocuarine chloride. In the last type, their properties vary from other alkaloids, owing to quaternary nature of nitrogen.
In the natural for, the alkaloids exist either in free form , as amine or as salt with acid or alkaloids N-oxides
The qualitative chemical tests used for detection of alkaloids are depend on the character of alkaloids to give precipitate as salts of organic acids or with compound of heavy metals like Hg, Au, Pt, etc.
The other methods proposed for classification of alkalids are as follows: -
Other modes of classification are: -
This method gives significance to the precious from which the alkaloids are biosynthesized in the plant. Hence, the varity of alkaloids with different taxonomic distribution and physiological activities can be brought under same group if they are derived from same procedure. E.g. all indole alkaloids from tryptophan are grouped together. The alkaloidal drugs are catagorised on the fact whether are derived from amino acid procedure as ornithine, lysine, tyrosine, phenylalanine, tryptophen, etc.According to this alkaloids are usually classified by their common molecular precursors, based on the metabolic pathway used to construct the molecule. When not much was known about the biosynthesis of alkaloids, they were grouped under the names of known compounds, even some non-nitrogenous ones (since those molecules' structures appear in the finished product; the opium alkaloids are sometimes called "phenanthrenes", for example), or by the plants or animals they were isolated from. When more is learned about a certain alkaloid, the grouping is changed to reflect the new knowledge, usually taking the name of a biologically-important amine that stands out in the synthesis process.
The extraction of alkaloids is based upon their basic character and solubility pattern. The normal procedures followed are to treat moistened drug with alkali so as to set free the base as it exists in salt form and then to separate free base with organic solvent. This is known as Stas Otto process. Through the method of extraction vary, gernally following procedure is applied for small scale extraction of alkaloids. First the plant is defatted with patrollium ether, eapatially in case of seed and leaf forms of drugs. Before applying this treatment the alkaloid should be tested for its solubility in patrolleum ether. Otherwise the drug should be pretreated with acid to be convert alkaloid into the salts. This happens in case of extraction of regotamine from ergot.
In the second stage the drugs may be extacted with polar solvents like water, ehanol, methnol aqueous alcohol mixture or with acidified aqueous solution. By this treatment alkaloidal salts are transferred to polar solvent. It also helps in remving pigments sugar and other organic constituents in the following stage. The alcohol solution is evaporated to a thick syrup and is subjected to partition between aqueous acid solution and an organic solvent. After continous extraction with organic solvent for some time the aqueous phase is made alkaline with ether sodium carbonate or ammonia. The basic aqueous solution is then extracted with convenient organic solvent followed by drying off alkaloid containing solution normally with sodium sulphate, filtered and evaporated to yield alkaloid residue.
The other method ment for extraction for alkaloids employs the treatment of drug with ammonia so as to covert the alkaloidal salt into their free bases such librated alkaloids in free base form are conveniently extracted with organic solvents like ether, benzene, chloroform, etc. the method is not useful for isolating alkaloids with quaternary nitrogen.
The further purification of crude extract of alkaloids is done by following ways which may however, vary for indivisual for alkaloids.
Purification or isolation of alkaloids from a plant is always difficult process
because an alkaloids bearing plant generally contains a complex mixture of
several alkaloids with glycoside organic acid also complicate the process.
Following steps are involved in isolation process.
Molecular formula of majority of Alkaloids is complex so very little achievement in their elucidation of structure. During 19th Century. Now general procedures for elucidation of structure of alkaloids are adopted.

The distinction between aldehyde and ketone is done by oxidation or reduction, also by NMR, IR, and UV techniques.




If alkaloids react with one molecule of methyl-iodide to form N-methyl derivative, it means secondary e.g.
The alkaloids are poisonous in nature, but when used in small quantities, exert useful physiological effects on animals and human beings and hence they have secured significant place in medicine. There exact role in nature and function in the plants, if any, are still a topic ambiguity. Only one aspect is clearly understood that they are synthesized by particular, stereospecific, many a time complicated, and energy consuming pathways and further they are metabolized to other alkaloidal or non-alkaloidal substances. Some of the predicted roles of alkaloids in the plants are discussed below: -

